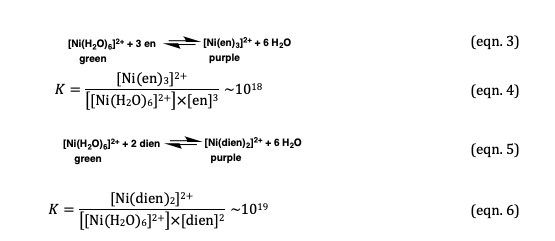![Why is [Ni(NH3)6]Cl2 paramagnetic but [Co(NH3)6]Cl3 is diamagnetic ? | Electron configuration, Coordination number, Crystal field theory Why is [Ni(NH3)6]Cl2 paramagnetic but [Co(NH3)6]Cl3 is diamagnetic ? | Electron configuration, Coordination number, Crystal field theory](https://i.pinimg.com/736x/9e/ad/9f/9ead9f774d9995ac47c672b48098f159.jpg)
Why is [Ni(NH3)6]Cl2 paramagnetic but [Co(NH3)6]Cl3 is diamagnetic ? | Electron configuration, Coordination number, Crystal field theory

Table 4 from Ni(NH3)2(NO3)2—A 3-D Network through Bridging Nitrate Units Isolated from the Thermal Decomposition of Nickel Hexammine Dinitrate | Semantic Scholar
86. The formation constant of Ni(NH3)6 is 6 ×10^8 at 25^° C. If 50 ml of 2.0 M NH_3, is added to 50 ml of 0.20M solution of Ni^2+, the concentration of

Inorganics | Free Full-Text | Ni(NH3)2(NO3)2—A 3-D Network through Bridging Nitrate Units Isolated from the Thermal Decomposition of Nickel Hexammine Dinitrate
![21 15. The oxidation state of Ni and NH, in [Ni(NH3)4]+2 (1) Ni = +2, NH, = 0 [RPMT 2001 (2) Ni = +1, NH, = - 1/6 (3) Ni = +1, NH, = + 1/6 (4) Ni = 0, NH, = +2 21 15. The oxidation state of Ni and NH, in [Ni(NH3)4]+2 (1) Ni = +2, NH, = 0 [RPMT 2001 (2) Ni = +1, NH, = - 1/6 (3) Ni = +1, NH, = + 1/6 (4) Ni = 0, NH, = +2](https://toppr-doubts-media.s3.amazonaws.com/images/4816097/e84878e9-2fe2-45ee-a89d-cd525e6ad65a.jpg)
21 15. The oxidation state of Ni and NH, in [Ni(NH3)4]+2 (1) Ni = +2, NH, = 0 [RPMT 2001 (2) Ni = +1, NH, = - 1/6 (3) Ni = +1, NH, = + 1/6 (4) Ni = 0, NH, = +2
2 – topic of research paper in Chemical sciences. Download scholarly article PDF and read for free on CyberLeninka open science hub. Thermal decomposition of polycrystalline [Ni(NH3)6](NO3)2 – topic of research paper in Chemical sciences. Download scholarly article PDF and read for free on CyberLeninka open science hub.](https://cyberleninka.org/viewer_images/173973/f/1.png)

![What is the IUPAC name of the complex [Ni(NH3)6]Cl2 | Coordination Chemistry Questions - YouTube What is the IUPAC name of the complex [Ni(NH3)6]Cl2 | Coordination Chemistry Questions - YouTube](https://i.ytimg.com/vi/GtBNjFWTE88/maxresdefault.jpg)





2. The... | Download Scientific Diagram Representation of the cubic structure of [Ni(NH3)6](NO3)2. The... | Download Scientific Diagram](https://www.researchgate.net/publication/269400063/figure/fig8/AS:667921089036294@1536256205990/Representation-of-the-cubic-structure-of-NiNH36NO32-The-constituent-atoms.jpg)

![Beautiful Crystals of [Ni(NH3)6]Cl2... - Chemistry is love | Facebook Beautiful Crystals of [Ni(NH3)6]Cl2... - Chemistry is love | Facebook](https://lookaside.fbsbx.com/lookaside/crawler/media/?media_id=676374066291248)